The Complete Code of Federal Regulations, Title 21, Food And Drugs, FDA Regulations, 2016 - Kindle edition by United States Government. Professional & Technical Kindle eBooks @ Amazon.com.

PDF) Food and Dietary Supplement Package Labeling—Guidance from FDA's Warning Letters and Title 21 of the Code of Federal Regulations | Tom Brody - Academia.edu


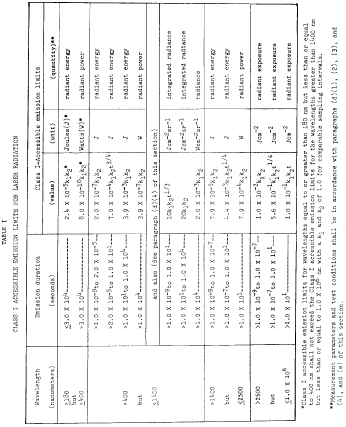

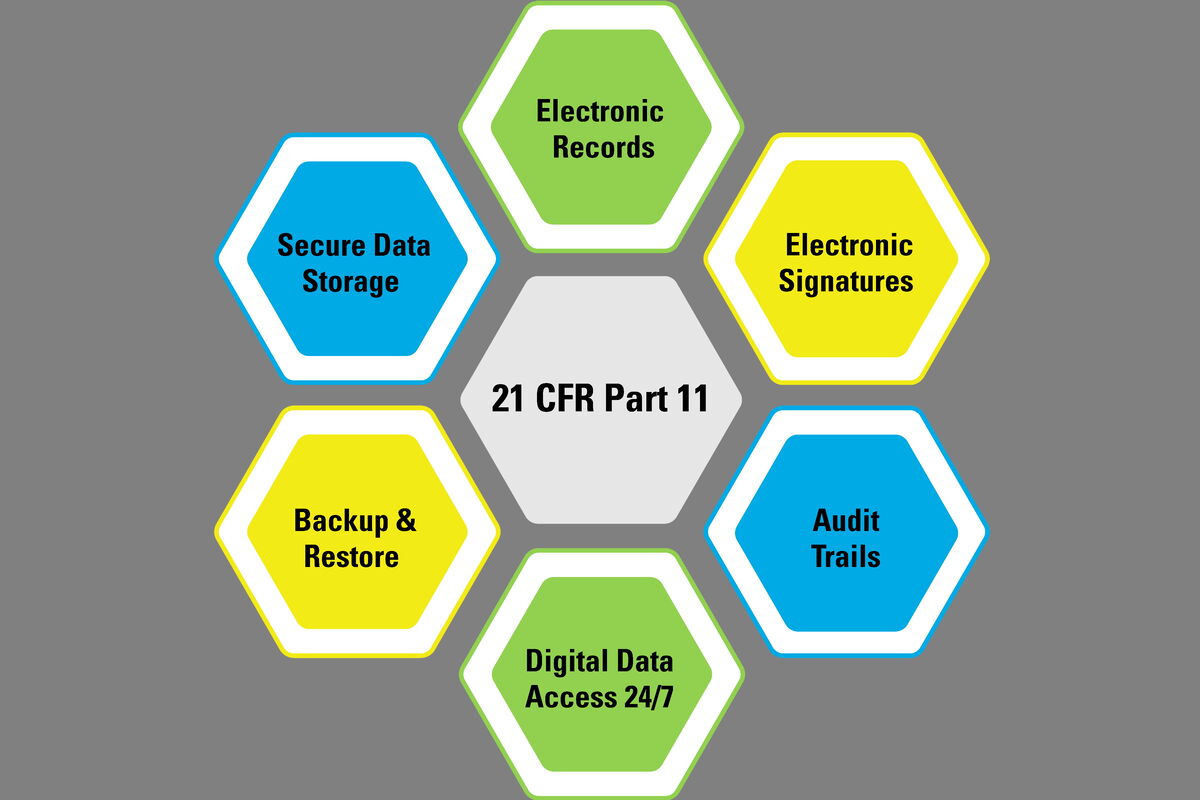
%20Complete%20Guide%20to%2021%20CFR%20Part%2011.png?width=1700&name=(cover)%20Complete%20Guide%20to%2021%20CFR%20Part%2011.png)